Growing the Future
of Medicine
From stem cells to new therapies — can we do more to treat disease and injuries?
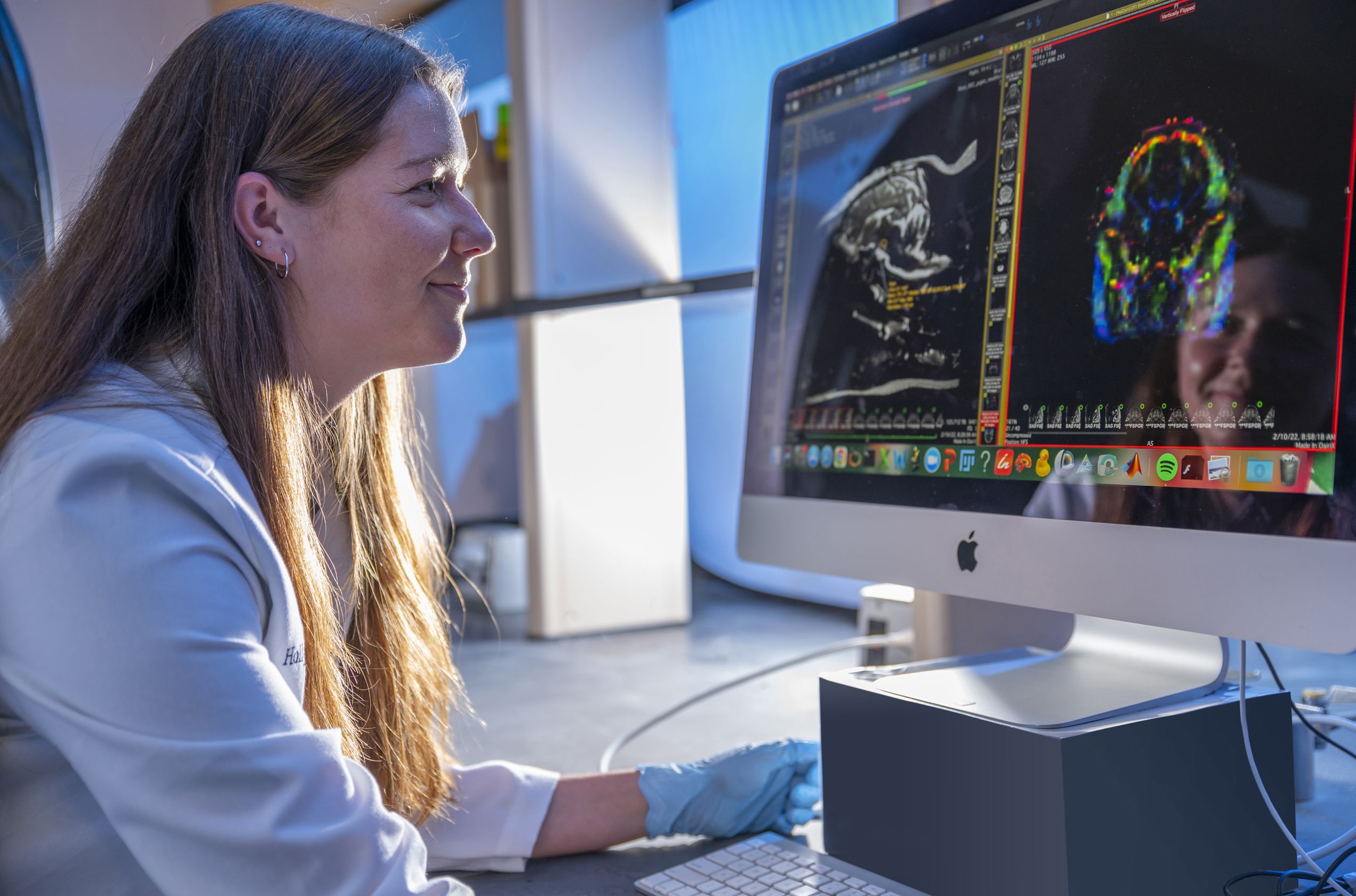
Undergraduate researcher and animal biology major Morgan Cunningham examines MRI images of a pig brain. (Photo by Dennis McDaniel)
Undergraduate researcher and animal biology major Morgan Cunningham examines MRI images of a pig brain. (Photo by Dennis McDaniel)
Steven Stice (right, pictured here with his wife, Tracey Stice) joined UGA in 1998 as the Georgia Research Alliance endowed chair and has shepherded the creation and growth of the Regenerative Bioscience Center. He also founded ArunaBio, a research-reagent-turned-therapeutics company. (Photo by Dorothy Kozlowski)
Steven Stice (right, pictured here with his wife, Tracey Stice) joined UGA in 1998 as the Georgia Research Alliance endowed chair and has shepherded the creation and growth of the Regenerative Bioscience Center. He also founded ArunaBio, a research-reagent-turned-therapeutics company. (Photo by Dorothy Kozlowski)
While current medicine can slow down the progression of many diseases, the relatively new field of regenerative biology uses stem cells and advanced therapies to treat or reverse the course of disease and injuries. The Regenerative Bioscience Center (RBC) at the University of Georgia has become a leader in this field.
The regenerative bioscience field developed in the early 2000s after scientists realized the potential of stem cells — a special kind of cells from which every other cell in our body develops — to treat many diseases, both in humans and animals.
Biotechnology expert Steven Stice already had broad experience in the field, including having founded a stem cell start-up, when he helped found the center in the College of Agricultural and Environmental Sciences in 2004.
“What was driving me then, and still does, are the huge unmet needs for reversing degenerative diseases, whether in the bone or in the brain,” said Stice, who has directed the RBC since its founding and serves as a D.W. Brooks Professor and Georgia Research Alliance Eminent Scholar Chair at UGA. “There’s also real opportunity to take things into clinical trials faster with these new therapies and apply them to actually start treating patients — human and animal.”
The RBC has been a pioneer in this field and was one of the first groups to show how neural stem cells can be grown in a lab. The RBC researchers were ultimately able to generate a therapy for stroke they hope will enter clinical trials in 2023. A key focus for the center is understanding the regenerative processes and mechanisms so that researchers, and ultimately medical practitioners, can guide the body to regenerate tissue that is lost in disease or injury, Stice explained.
To support this work, RBC researchers are developing new ways to test and evaluate diseases. This all requires a highly interdisciplinary and collaborative approach, which many RBC researchers feel is the key to success in this area.
As researchers at RBC and around the world have dug deeper into the field, its complexity has become apparent.
“I think, at the beginning, we had the fanciful thought that all we had to do to cure disease is make a nerve cell and introduce it into the brain,” said Stice. “Now we know that it takes much more than that. So, a lot of the work at the RBC is around what helps support the cells once they get there so they can do the things that they need to do.”
“It takes scientific teams of diverse talents to develop solutions to complex biomedical injuries and diseases and then translate them into real treatments quickly and efficiently.”
— Steven Stice

Stice’s colleague and collaborator, John Peroni, who was recently appointed the first Dr. Steeve Giguère Memorial Professor at the UGA College of Veterinary Medicine, agrees.
“Much has been learned about stem cell biology over the years and this knowledge has led to a departure from thinking that these cells replace injured tissue,” Peroni said. “It’s more likely that stem cells, specifically mesenchymal stem cells, release factors that guide the inherent tissue recovery processes to optimize healing.”
Combining his knowledge in large animal veterinary surgery with research in stem cells, Peroni and other veterinary researchers have been employing stem cells to treat tendon and ligament injuries, which are very common and debilitating for horses.
“The end result of stem cell treatment is improved organization of the healed tendon so that, compared to an untreated tendon, scar tissue formation is minimized, resulting in a more organized tendon matrix that is better suited to sustain loads and therefore less prone to re-injury,” he said.
While stem cells are a central part of regenerative biology research, it is possible that they may not be needed to unlock some of the promises of regenerative medicine. The key could lie not only in the stem cells, but in the small nano-sized bubbles that all cells produce, as well as other biological products in our body, Stice said.
For example, Peroni has found that a blood-derived product made from concentrating platelets — platelet lysate — can perform as an antibiotic on a variety of bacteria.
“We found that platelet lysate significantly decreases bacterial growth,” said Peroni. “We realized this when we provided platelet lysate to one of the clinicians in our hospital to promote healing on skin grafts placed on an injured horse. The graft had a surface infection, but after topically applying the lysate for a few days, the infection disappeared, which got us really excited about exploring this further.”
Peroni is now exploring how to apply lysate as an antibiotic in different veterinary settings to learn if it can be a valuable substitute for traditional antibiotics to treat infections. If successful, this could present a huge advantage in infection control because it appears that bacteria cannot develop resistance to the lysate.
Jamise Lee, an assistant professor in physiology and pharmacology and a researcher at RBC, is interested in how a type of innate immune cells present in human blood — called natural killer cells — could have wider applications in neurodegenerative diseases.
When a cell becomes cancerous, is infected by a virus or stops working as it ages, natural killer cells attack the cell and clear it from our bodies. Using a mouse model of Parkinson’s, Lee also found that the disease becomes worse if natural killer cells are removed. This could have applications to find markers for early diagnosis and possible therapies to treat Parkinson’s disease.
“For years, the study of neurodegenerative diseases was focused on the neuron aspect,” said Lee. “More recently, people have become more interested in how the immune system could interact or intercommunicate with the brain.”
RBC scientist Hongxiang Liu has been studying taste buds for more than two decades, identifying stem cells that eventually become taste buds and generating understanding of what happens at the molecular level to lead to specific cells.
The body is constantly generating new taste bud cells, which have a lifespan of only 12 days on average. Stem cells become progenitor cells, which develop into specialized cells in the body. Liu has shown that not all types of taste bud cells come from the same type of progenitor cells — bitter and sweet taste bud cells may develop from different progenitors than sour taste cells. By understanding how these different taste buds develop, Liu hopes this work will lead to therapies that help restore taste.
“Many of us take taste for granted,” said Liu. “But the loss of taste often compromises quality of life and could help us understand diseases better.”
RELATED READING

Did you enjoy this story?
Check out recent issues of the Almanac for more great stories like this one.